Cu
What is copper
Ag
What is silver
K
What is potassium
Al
What is aluminum
H
What is hydrogen
The Atomic Number is equal to the...
What is the number of protons and electrons
All of the elements except for Hydrogen have this number of electrons on the first, inner most shell/ring.
What is 2?
Neutrons have this charge.
What is no charge or neutral charge?
This family is gases that are colorless, odorless and very stable.
What are Noble Gases?
Draw the Bohr Model of Phosphorous
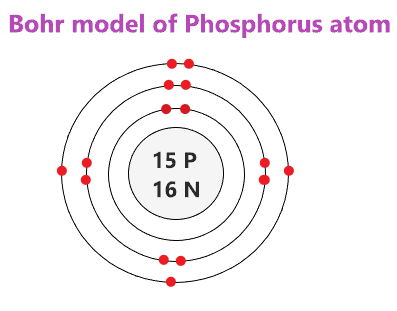
The electrons in the outer most shell are known as?
What are valence electrons
The subatomic particles found in the nucleus of an atom.
What are protons and neutrons?
True or False:
Electrons are positively charged.
What is False
The elements on the far left of the periodic table have high thermal and electrical conductivity, luster, ductility and malleability
What are the Alkali Metals?
Draw a Bohr Model of Oxygen.
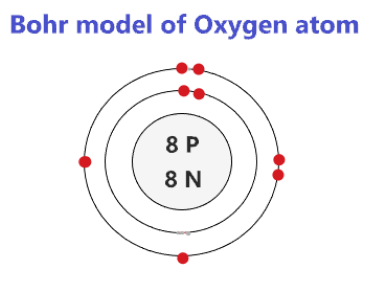
To find the number of neutrons an element has you need to...
What is subtract the atomic number from the atomic mass.
Group 18 elements are extremely nonreactive nonmetals known as this.
What are noble gases?
True of False:
Protons are positively charged
What is True
This family tends to be fairly toxic, highly reactive are are all non-metals.
What are Halogens?
The two models still used most often today.
What are the Bohr Model/Planetary Model and the Quantum Model/Cloud Model?
Give an example of a heterogenous mixture.
Tator Tot Hotdish, Chili, Burrito, Italian Dressing, etc.
The first energy shell is located where in relation to the nucleus.
Where is closest to the nucleus?
Name the following about NaCl.
# & name of Elements
# Molecules
# of Atoms (total and per element)
2 Elements (Sodium and Chlorine)
1 molecule (1 structure)
1 atom of Sodium, 1 atom of Chlorine, 2 total atoms
The family that is made up of metals, mostly solid and include the two rows that look like they're under the periodic table the Lanthanides and the Actinides.
What are Transition Metals?
Only one type of compound present
What is a pure compound?